Translate this page into:
Pseudoaneurysm of Uterine Artery: A Rare Cause of Secondary Postpartum Hemorrhage, Managed with Uterine Artery Embolisation
Address for correspondence: Dr. Ainharan Raveendran, Department of Obstetrics and Gynaecology, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh-160 012, India. E-mail: rainharan@yahoo.co.in
-
Received: ,
Accepted: ,
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article was originally published by Medknow Publications & Media Pvt Ltd and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Uterine artery pseudoaneurysm is a rare cause of secondary postpartum hemorrhage but is potentially life-threatening and can occur after caesarean section (c-section) or a hysterectomy. A 28-year-old woman who developed secondary postpartum hemorrhage after c-section was diagnosed to have pseudoaneurysm from the left uterine artery on ultrasound (US) and computed tomography (CT) scan. She was treated with coiling of the pseudoaneurysm with stainless steel coil via selective catheterization of the uterine artery. The procedure was uneventful and the pseudoaneurysm was successfully obliterated. Angiographic embolization is a safe and effective method for treating postpartum hemorrhage due to pseudoaneurysm in hemodynamically stable patients. Therefore, it should be considered as a treatment option before resorting to surgery, in appropriately selected cases.
Keywords
Angiography
Doppler
secondary postpartum hemorrhage
uterine artery embolization
uterine artery pseudoaneurysm
INTRODUCTION

A pseudoaneurysm is an extra-luminal collection of blood with turbulent flow that communicates with the parent vessel through a defect in the arterial wall. The development of an arterial pseudoaneurysm is a rare but reported complication of pelvic surgery, vascular trauma during c-section or after uterine curettage. After hematoma formation, there is central liquefaction that leaves a cavity with turbulent blood flow, as a result of persistent communication between the parent artery and the hematoma. The absence of a 3-layer arterial wall lining the pseudoaneurysm differentiates it from a true aneurysm, which is less common than a pseudoaneurysm.[1]
Pseudoaneurysm of the uterine artery is an uncommon cause of delayed postpartum hemorrhage following caesarean or vaginal delivery and is potentially life threatening. Typically, the lesions are discovered because the patients have symptoms related to delayed rupture of the pseudoaneurysm, causing hemorrhage.[2] A pseudoaneurysm may be asymptomatic, may thrombose, or may lead to distal painful embolization. The risk of rupture is proportional to the size and intramural pressure. Diagnosis is usually based on both Doppler sonography and arteriography.[3] Transcatheter uterine artery embolization (UAE) has emerged as a highly effective technique for controlling obstetric and gynaecologic hemorrhage, including that from pseudoaneurysms.
We report a case of uterine artery pseudoaneurysm presenting with secondary postpartum hemorrhage 3 weeks after c-section delivery and managed successfully with coil embolization.
CASE REPORT
A 28-year-old gravida 2, para 1 was transferred to our institution 19 days post operation with symptoms of excessive bleeding per vaginum. She had undergone an elective c-section for suspected macrosomia. She was apparently asymptomatic for 15 days post operation. She later developed excessive bleeding per vaginum, high grade fever, and was readmitted. On abdominal examination, the c-section scar was found to be healthy and no abnormality was detected. She was bleeding per vaginum. The uterus was bulky and the cervical os was closed. Her hemoglobin level was 3.8 g/dl. She was stabilized with crystalloids, five units packed red blood cells, and started on broad spectrum antibiotics.
Transvaginal ultrasonography with color Doppler confirmed a postpartum uterus. There was no evidence of residual placental tissue in the uterine cavity and endometrial thickness was 7 mm. A hypoechoic lesion measuring 2 cm × 1.5 cm [Figure 1a] was detected in the isthmic region of uterus and power Doppler revealed blood flow within it [Figure 1b]. Color flow Doppler sonography showed yin and yang blood flow pattern within the body of pseudoaneurysm.
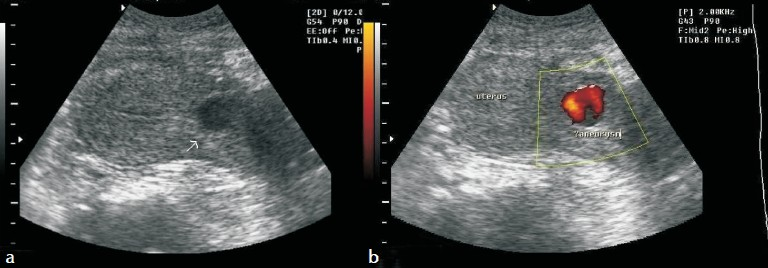
- (a) Gray-scale ultrasound of the pelvis shows a hypoechoic lesion in the anterior wall of uterus. (b) Power Doppler evaluation of the uterus demonstrates blood flow within this hypoechoic lesion.
Computed tomography angiogram was done with non-ionic contrast showed early contrast filling of the lesion within the uterus [Figure 2a, b]. Maximum intensity projection (MIP) and Volume rendered (VR) images nicely demonstrated the pseudoaneurysm in relation to the uterine artery [Figure 2c, d].
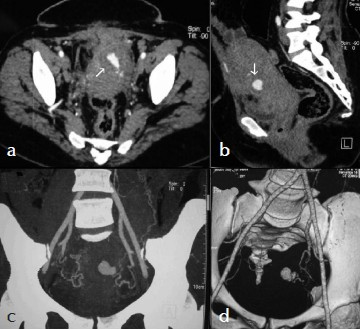
- (a) Axial and (b) Sagittal MPR CT Angiogram images show contrast filling the pseudoaneurysm (arrow) within the uterus. (c) MIP and (d) VR images show a pseudoaneurysm in relation to the left uterine artery.
To preserve the fertility in this young patient, a transcatheter arterial embolization of this pseudoaneurysm was planned. She underwent digital subtraction angiography. Arteriography revealed a pseudoaneurysm from the terminal part of the left uterine artery, in addition the left uterine artery was tortuous and was hypertrophied [Figure 3a, b]. Left uterine artery was selectively embolized with mixture of gelfoam and contrast media followed by 2 stainless steel coils 4 mm in diameter [Figure 4a]. The right uterine artery was also tortuous and hypertrophied and was embolized with gel foam. A post embolization angiographic study was performed to ensure the complete occlusion of the vessels [Figure 4b]. Follow-up color Doppler US showed aneurysmal cavity filled with echogenic content with no evidence of blood flow [Figure 4c], except for pain in the abdomen that was managed with analgesics.
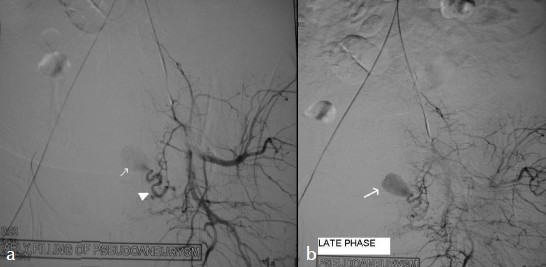
- Selective left internal iliac angiogram: (a) Early and (b) late phase shows the pseudoaneurysm (arrows), arising from the left uterine artery (arrowhead) in addition the hypertrophy of the left uterine artery can also be appreciated.
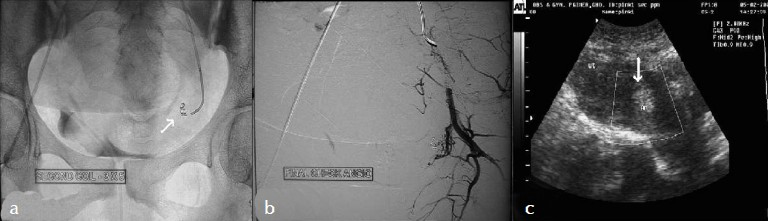
- (a) Embolization coils (arrow) within the pseudoaneurysm. (b) Selective left internal iliac angiogram (Postembolization) using gelfoam and embolization coils show complete obliteration of the pseudoaneurysm. (c) Follow up color Doppler sonography shows no evidence of blood flow within the pseudoaneurysm.
DISCUSSION
Postpartum hemorrhage remains one of the major causes of maternal mortality. Secondary postpartum hemorrhage is defined as excessive bleeding starting any time from 24 hours after delivery up to 6 weeks postpartum and most commonly occurring between 8 and 14 days postpartum. Common causes include retained products of conception, subinvolution of the placental bed, and endometritis.[4] Rare causes include pseudoaneurysm of uterine artery, arteriovenous malformations, and choriocarcinoma. When the more common causes have been excluded, pelvic angiography may be performed. Uterine artery embolization can be carried out to control hemorrhage. In 1979, Brown et al., reported the first case of selective arterial embolization used successfully to treat an extrauterine pelvic hematoma after three failed surgical attempts to control the bleeding.[5] Since then, arterial embolization has been used successfully to control postpartum bleeding from uterine atony, placenta accreta, and vulvar and vaginal hematomas. The efficacy and safety of selective arterial embolization of uterine arteries was evaluated by Pelage et al., in women with delayed secondary postpartum hemorrhage. In their series of 14 women, pseudoaneurysms of the uterine artery were found in 2 women.[6] Immediate resolution of external bleeding was observed after embolization. In this series, no complications related to this invasive treatment were found. Other authors have described complications, including muscle pain and bladder necrosis.[7]
A true aneurysm has all three layers of arterial wall, whereas pseudoaneurysm does not have all the three layers of arterial wall. The differential diagnosis of pseudoaneurysm includes acquired arteriovenous malformations (AVMs), arteriovenous fistulas, and direct vessel rupture. AVMs are characterized by multiple communications of varying sizes between arteries and veins, which can be congenital or acquired. Congenital uterine AVMs are due to abnormality in the embryologic development of primitive vascular structures, whereas acquired AVM's consist of multiple small arteriovenous fistulas between intramural arterial branches and the myometrial venous plexus. Acquired AVM's occur more commonly following D and C, uterine surgery, or trauma to the uterus. Color flow Doppler demonstrates to-and-fro sign in the neck of the pseudoaneurysm and yin-yang sign in the body of the pseudoaneurysm. AVM's are characterized by marked aliasing on color flow Doppler and arterialized venous flow on spectral Doppler evaluation.
In a small series of women,who underwent embolotherapy for obstetric hemorrhage, all 3 women who attempted conception after embolization were successful. Of the 3 women, 2 underwent bilateral uterine artery embolization.[8] Our patient developed a pseudoaneurysm after 2 weeks of c-section delivery. Treatment was by angiographic embolization of uterine arteries with gelfoam and embolization coils. In a series of women, Rosenthal et al., observed angiographic arterial embolization was shown to be the most useful clinical tool in the management of post operative vaginal hemorrhage.[9] Angiographic embolization has the advantages of decreased morbidity, ability to localize the bleeding site and provide a more distal occlusion than surgical ligation, and preservation of future fertility compared to hysterectomy. Burchell demonstrated that bilateral internal iliac artery ligation was more effective in reducing the pulse pressure than unilateral ligation.[10] It is possible that the redistribution and redirection of blood or hypoxia-induced neovascularization allows bleeding to occur from the contralateral side after unilateral embolization. Inadequate embolization of a pseudoaneurysm due to extrauterine feeding arteries, such as the internal pudendal artery, ovarian artery, inferior epigastric artery, or contralateral uterine artery leading to embolization failure can occur.[1] Hence, bilateral uterine embolization is safe and more advantageous than unilateral embolization.
We conclude that in a woman with unexplained vaginal bleeding after c-section delivery, pseudoaneurysm is a potentially life-threatening complication and should be considered in the differential diagnosis of secondary postpartum hemorrhage. Although data are scant, bilateral uterine artery embolization for obstetric hemorrhage appears to have no increased deleterious effect on future fertility and is more effective when compared to unilateral embolization.
Source of Support: Nil
Conflict of Interest: None declared.
Available FREE in open access from: http://www.clinicalimagingscience.org/text.asp? 2011/1/14/76692

