Translate this page into:
Rare case of atypical Dejerine syndrome in a child

*Corresponding author: Lee K. Rousslang, Department of Radiology, Tripler Army Medical Center, Honolulu, Hawaii, USA. lee.k.rousslang.civ@mail.mil
-
Received: ,
Accepted: ,
How to cite this article: Rousslang LK, Reitz JT, Rooks E, Wood JR. A case of medial medullary infarction leading to atypical Dejerine syndrome in a child. J Clin Imaging Sci 2020;10:2.
Abstract
Medial medullary syndrome (aka Dejerine syndrome) is a rare condition that develops following infarction of the medial medulla and is classically defined by the presence of Dejerine’s triad of contralateral weakness in upper and lower extremities, contralateral hemisensory loss of vibration and proprioception, and ipsilateral tongue weakness. It is typically caused by occlusion of the vertebral artery or one of its branches. We report the case of a 6-year-old girl who suffered a medial medullary infarction, and she was diagnosed with atypical Dejerine syndrome. Medial medullary infarct leading to atypical Dejerine syndrome has not been reported in this young of a patient in the literature to date.
Keywords
Medial medullary infarction
Pediatric stroke
Dejerine syndrome
INTRODUCTION
Ischemic stroke in children is rare, with an estimated incidence of 0.6–7.9 cases per 100,000 children per year.[1] Medial medullary infarctions (MMIs) are even rarer and comprise <1% of all ischemic strokes in posterior circulation.[2] MMI leading to Dejerine syndrome can prove challenging to detect, even with the widespread use of diffusion-weighted imaging (DWI), due to its location in the brainstem.
CASE REPORT
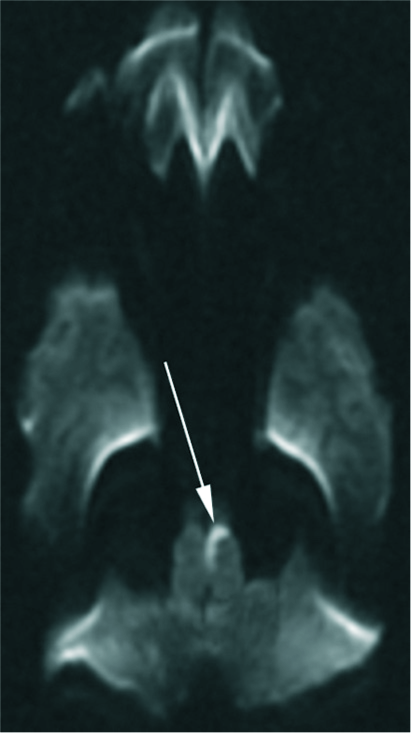
A 6-year-old girl presented to the emergency department with the right-sided weakness, right-sided proprioception deficits, vertigo, nystagmus, and a headache with emesis. Her symptoms began as a headache, vertigo (described as “the room is spinning”), and an episode of emesis that started right after swinging on the monkey bars at school. There was no fall or loss of consciousness. The following morning, her parents noticed that she had right-sided weakness and the inability to walk and took her to the emergency department. On examination, she displayed marked right-sided weakness in her upper and lower extremities with facial sparing, a concomitant loss of proprioception in the same distribution, and a vertical nystagmus with a rotational component. Initial computed tomography (CT), magnetic resonance imaging (MRI), and lumbar puncture were normal. She was then transported to our hospital for further care. Her symptoms persisted for 2 days, prompting a repeat MRI [Figure 1] that demonstrated acute infarction of the left medial medulla, which, in conjunction with her history, supported the clinical diagnosis of an atypical Dejerine syndrome.

- A 6-year-old girl presented with right-sided weakness, a right-sided proprioception loss, vertigo, nystagmus, headache, and emesis. Axial diffusion-weighted imaging (DWI) (a) demonstrates the linear, medial medullary infarct that is bright on diffusion and dark on the corresponding apparent diffusion coefficient (ADC) map (b). The lesions were also present on coronal DWI (c) and ADC (d). Axial fluid- attenuated inversion recovery image (e) demonstrates mild increased signal intensity in the left medial medulla. The findings are consistent with acute infarction of the medial margin of the left half of the medulla. In conjunction with history, these findings support the clinical diagnosis of medial medullary syndrome aka Dejerine syndrome.
On retrospective review, the initial MRI done at the outside hospital [Figure 2] demonstrated significant artifact at the skull base, and as a result, a small diffusion restriction may have been overlooked.
- A 6-year-old girl presented with right-sided weakness. The initial axial diffusion-weighted imaging was done at an outside hospital, but due to significant skull base artifact, a small area of restricted diffusion may have been overlooked.
Given the initial negative imaging on CT and MRI, this case serves as a reminder of the importance of avoiding inattentional (aka “blind spot”) bias, particularly with regard to posterior circulation infarcts.
DISCUSSION
Infarction of the medial medulla, leading to medial medullary syndrome (Dejerine’s), is a rare condition that comprises <1% of all ischemic strokes in posterior circulation.[2] It is classically defined by the presence of Dejerine’s triad of contralateral weakness in upper and lower extremities (with facial sparing) due to pyramidal tract involvement, contralateral hemisensory loss of vibration and proprioception due to medial lemniscus involvement, and ipsilateral tongue weakness, due to CN12 nucleus involvement.[3] This syndrome results from occlusion of the vertebral artery or one of its medial branches.
The largest clinical study of MMI to date examined 86 patients with MRI-proven MMI and found considerable variability in symptoms, with the most common being hemiplegia (91%), vertigo/dizziness (59%), impaired vibratory sense (56%), impaired proprioception (48%), nystagmus (44%), dysphagia (29%), nausea/vomiting (16%), and headache (10%).[4] Despite the eponymously named Dejerine triad of symptoms, ipsilateral tongue weakness is a rare finding (<3%).[4,5] This is likely because tongue weakness is due to lateral medullary involvement and is not seen in the overwhelming majority of patients with small, paramedian infarcts such as ours.[6]
Although DWI is sensitive to acute stroke approximately 92% of the time, the detection rate of posterior circulation strokes may only be half as high as that in anterior circulation.[7,8] One possible explanation for this is that the brainstem is a relative “blind spot” for radiologists.[9] Compared to cerebral infarcts, brainstem lesions are often harder to detect because they are smaller, may lack pronounced mass effect, and have more densely compact anatomy, with close proximity of vascular, bony, neural, and soft tissue structures.[9] Moreover, medulla oblongata infarctions, as seen in our patient, are the least detectable, even compared with other brainstem regions.[10]
Although DWI is the most sensitive method of detecting acute ischemic stroke, cross-sectional CT angiography and CT perfusion (CTP) have emerged as useful adjuncts in evaluating posterior circulation infarcts.[11,12] CTP can further improve infarct detection and identify salvageable brain tissue (penumbra) more rapidly than MRI alone. As a result, it can potentially prolong the window for intravascular therapy and improve functional outcomes.[11,12] CTP is an especially useful adjunct for evaluating posterior fossa infarcts, which are notoriously difficult to detect.[12]
CONCLUSION
MMIs are very rare and comprise <1% of strokes in children.[2] Their diagnosis can be especially difficult, as the brainstem is a relative “blind spot” for many radiologists.[9] In this case, the diagnosis was also complicated by a significant skull base artifact on the initial MRI. Understanding of classic presentations, such as Dejerine syndrome, can help localize findings on imaging.
Acknowledgment
The views expressed in this manuscript are those of the authors, and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the United States Government. The authors have no financial, personal, or other vested interests in the information contained within this document.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Cerebrovascular disorders in children. Curr Neurol Neurosci Rep. 2004;4:129-38.
- [CrossRef] [PubMed] [Google Scholar]
- Medial medullary stroke: Report of seven patients and review of the literature. Neurology. 1997;48:882-90.
- [CrossRef] [PubMed] [Google Scholar]
- Stroke and cerebrovascular diseases. In: Ropper AH, Samuels MA, Klein JP, Prasad S, eds. Adams and Victor's Principles of Neurology (11th ed). New York: McGraw-Hill; Available from: http://www.accessmedicine.mhmedical.com/content.aspx?bookid=1477§ionid=196762701. [Last accessed on 2019 Oct 06]
- [Google Scholar]
- Medial medullary infarction: Clinical, imaging, and outcome study in 86 consecutive patients. Stroke. 2009;40:3221-5.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical study of 27 patients with medial medullary infarction. J Stroke Cerebrovasc Dis. 2017;26:2223-31.
- [CrossRef] [PubMed] [Google Scholar]
- Medial medullary syndrome: Report of 18 new patients and a review of the literature. Stroke. 1995;26:1548-52.
- [CrossRef] [PubMed] [Google Scholar]
- Sensitivity of diffusion-and perfusion-weighted imaging for diagnosing acute ischemic stroke is 97.5%. Stroke. 2015;46:98-101.
- [CrossRef] [PubMed] [Google Scholar]
- False-negative diffusion-weighted MR findings in acute ischemic stroke. AJNR Am J Neuroradiol. 2000;21:1434-40.
- [Google Scholar]
- Quality initiatives: Blind spots at brain imaging. Radiographics. 2009;29:1877-96.
- [CrossRef] [PubMed] [Google Scholar]
- MRI characteristics of acute and subacute brainstem and thalamic infarctions: Value of T2-and diffusion-weighted sequences. J Neurol. 2002;249:33-42.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of CT perfusion in the setting of cerebral ischemia: Patterns and pitfalls. AJNR Am J Neuroradiol. 2010;31:1552-63.
- [CrossRef] [PubMed] [Google Scholar]
- Evaluation of perfusion CT and TIBI grade in acute stroke for predicting thrombolysis benefit and clinical outcome. J Neuroradiol. 2009;36:131-7.
- [CrossRef] [PubMed] [Google Scholar]

